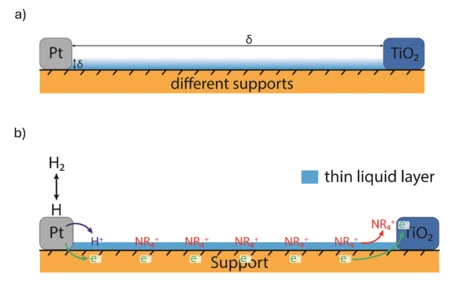
a) Scheme of a model system for the investigation of hydrogen transfer phenomena on thermocatalysts. Modification of the distance between catalytic sites, composition and thickness of solvation layer as well as the nature of the support is possible. b) Example for a thermochemical reaction interpreted as an electrochemical half-reaction in the case of a system with a non-protic solvent containing a non-hydronium cation.
Our group investigates charge transfer processes at the surface of thermocatalysts to gain a deeper understanding of the underlying kinetics. Since many thermocatalysts involve a multitude of elementary reaction steps, it is often challenging to clearly determine the contribution of each step to the overall kinetics. Moreover, many thermochemical reactions can be regarded as electrochemical half-reactions, in which electron and ion transport occur simultaneously. While the overall kinetics of such systems are often well described, a more detailed step-by-step analysis can provide valuable mechanistic insights.
By employing model systems and various spectroscopic techniques, we aim to disentangle these contributions. Particular attention is given to the influence of temperature, solvent, and support composition, in order to identify the rate-determining steps within each system. This knowledge enables us to draw further conclusions about fundamental transfer mechanisms and to guide the optimization of catalytic materials. As an initial focus, our research centers on hydrogenation reactions such as the CO₂ hydrogenation to methanol over Cu/ZnO/Al₂O₃ catalysts.
Contact: katha.blank@tum.de
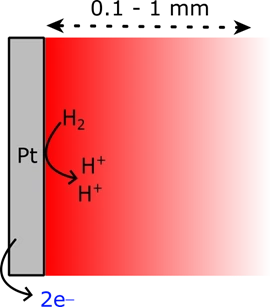
Interfacial pH swing during hydrogen oxidation reaction (HOR)
Proton‐coupled electron transfer (PCET) reactions underpin almost all electrochemical energy transformations, including H2 and O2 evolution, CO₂ reduction, and N₂ reduction. Since PCET involves the consumption of hydronium at the electrode–electrolyte interface, its thermodynamic driving force is explicitly pH dependent. At extreme pH, the high concentration of H⁺ or OH⁻ effectively buffers interfacial hydronium or hydroxide flux, thus resisting changes in local pH. In contrast, under near‐neutral conditions (pH 5–9), even modest proton transfer rates substantially perturb the interfacial pH, dynamically coupling the applied potential (referenced to a pH‐independent standard) with the fluctuating proton activity. This coupling complicates kinetic analyses of PCET and restricts straightforward interpretation of voltammetry, resulting in a paucity of kinetic data on intermediate pH PCET reactions.
To overcome this limitation, we are developing voltametric methods capable of accounting for interfacial pH swing without the use of pH buffering agents (that can convolute the intrinsic PCET kinetics). Through this approach, we aim to isolate and characterise the genuine kinetics of PCET reactions under unbuffered, near-neutral pH conditions.
Contact: antony.james@tum.de
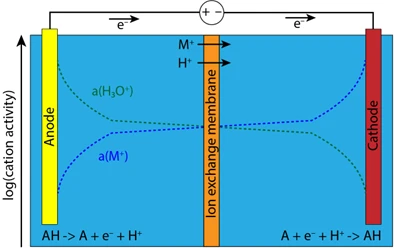
Ion transport in typical electrochemical systems. The net electron transport is accompanied by ion transport, often leading to ion activity gradients across the electrochemical cell.
Our lab takes a deeper look at ion transport phenomena in electrochemical systems, which is a critical yet often overlooked factor in the performance of electrochemical energy technologies, i.e. batteries, fuel cells, and electrolysers. The movement of electrons across electrochemical interfaces is usually fast and comparatively well-understood, while the transport of ions is largely overlooked. Device performance and stability is usually limited by slow ion movement. We develop a new methodology for the measurement of ion transport across electrochemical systems under operating conditions. By isolating contributions of diffusion, migration, and convection, we are able to obtain detailed insights into how ion gradients are established in electrochemical systems, particularly in reactions involving proton-coupled electron transfer (PCET).
Beyond aqueous liquid-phase systems, we will also study differential ion transport kinetics in solid-state materials such as polymeric and solid-state ion-selective membranes. By establishing our approach to non-aqueous electrolytes, we can probe the impact of solution non-idealities in impacting the performance of lithium- or sodium-ion batteries. Overall, our goal is to develop methods to spatially and temporally resolve ion transport phenomena in a wide range of electrochemical systems, providing insight into improved electrochemical technologies.
Contact: florian.musialek@tum.de