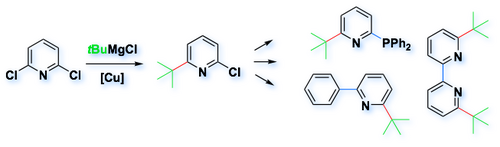
B-1 - Catalytic tert-alkyl cross-coupling
We have developed a catalytic cross-coupling reaction for tertiary alkyl groups, which can be applied to the selective synthesis of bulky heterocyclic building blocks (Angew. Chem. Int. Ed. 2008, 47, 8246).
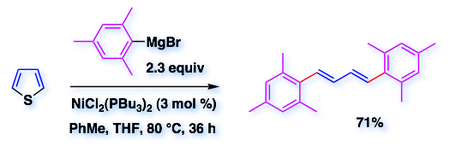
B-2 - Desulfurative cross-coupling
The use of alternative leaving-groups in cross-couplings opens surprising short-cuts to certain targets:
• We have improved a cross-coupling of thiophene with Grignard reagents based on work by Wenkert (1984) and extended the methodology to symmetric 1,4-diarylbutadienes, which are now available in one step from simple starting materials (Adv. Synth. Catal. 2010, 352, 2411).
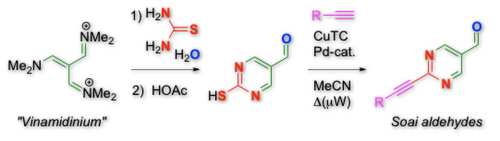
• a short-cut was also achieved in a new synthesis of Soai type aldehydes (highly interesting substrates for studying asymmetric atuocatalysis): the condensation of Arnold's vinamidinium-salt with thiourea gives a precursor, from which the targets are reached by direct, desulfurative alkyne coupling (Org. Lett. 2014, 16, 1282):