A-1 - Catalytic anti-Markovnikov hydration of terminal alkynes
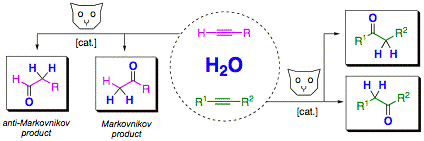
The catalytic anti-Markovnikov addition of water to terminal alkynes yields synthetically useful aldehydes in a single step with atom-economy (review: Synthesis 2007, 1121). The reaction is thus highly interesting for organic synthesis. We have, over the past few years, developed highly active and easy to handle catalyst systems for this reaction, which have enabled first synthetic applications in multi-step synthesis.
New catalysts for anti-Markovnikov hydration of terminal alkynes
We have introduced an easy-to-handle and highly active in situ catalyst for anti-Markovnikov hydration of terminal alkynes that has performed favorably in several syntheses (Org. Lett. 2006, 8, 5853). This catalyst has been prominently applied in total synthesis by the Fürstner group at MPI Mülheim (Angew. Chem. Int. Ed. 2011, 50, 8739).
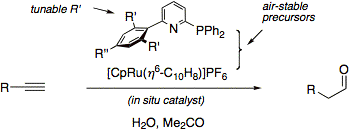
In the meantime, a new and improved in situ catalyst for anti-Markovnikov hydration of terminal alkynes has been developed that consists of CpRuCl(PPh3)2 and the ligand ISIPHOS, in aqueous acetone (J. Am. Chem. Soc. 2011, 133, 8138).
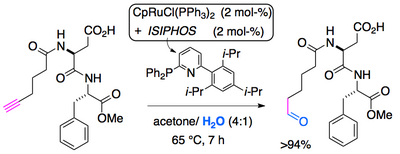
anti-Markovnikov hydration in organic synthesis
- iterative synthesis of oligo-1,4-diols (Synthesis 2007, 2809)
- catalytic anti-Markovnikov-hydration of N-tosyl-propargylamides to β-tosylamido-aldehydes (J. Org. Chem. 2007, 72, 5704)
- application in total synthesis: A. Fürstner et al, Angew. Chem. Int. Ed. 2011, 50, 8739)
- iterative synthesis of aldehydes or alkynes for introduction of 13C labels into alkyl chains (A. Brunner, in preparation)
A-2 - Catalytic hydroalkoxylation of alkenes
Additions of alcohols to alkenes are more difficult to catalyze and generally less investigated than corresponding reactions of alkynes. There are two mechanistic types of such reactions: with electron-poor alkenes (Michael acceptors), base-catalyzed conjugate additions can be realized (oxa-Michael reactions), in which the low nucleophilicity of the alcohols is limiting the application range.
• Our work on asymmetric catalytic oxa-Michael cyclizations
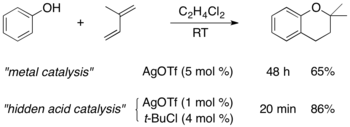
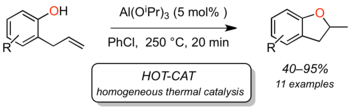
The second type is the classical electrophilic addition of alcohols/water to non-activated (but usually electron-rich) alkenes, which proceds acid-catalyzed via carbenium ions. The regioselectivity of such reactions is determined by the Markovnikov rule, whereas enantioselectivity cannot yet be controlled. Progress in Lewis acid catalyzed hydroalkoxylation has been reported, but we have shown that several of those reactions show characteristics of "hidden Brønsted acid" catalysis, and they are not enantioselective (J. Org. Chem. 2011, 76, 9353; review: Top. Organomet. Chem. 2010, 31, 123).