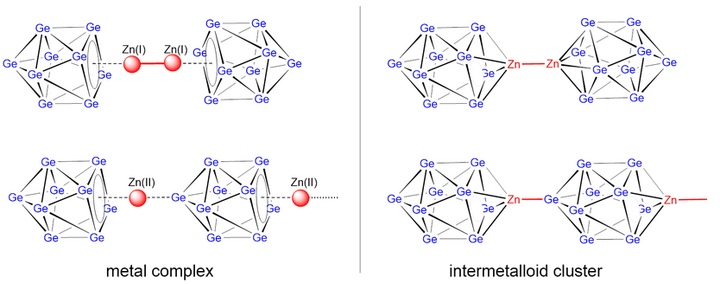
π–complex or intermetalloid cluster?
Zn atoms serve for both. Either covalent coupling of two Zn vertex atoms or Lewis-acid Lewis-base interactions leads to novel intermetalloid clusters with Zn.
The reactions of Zn(I) compounds of the type Zn2L2 with solutions of the Zintl phase K4Ge9 in liquid ammonia or ethylenediamine (en) lead for L = [HC(PhN=PPh2)]– under retention of the Zn–Zn bond to the anion [(h4–Ge9)Zn–Zn(h4–Ge9)]6– representing the first complex with a Zn–Zn unit carrying two cluster entities. The trimeric anion [(h4–Ge9)Zn{m2(h1: h1Ge9)}Zn(h4–Ge9)]8– occurs as side product, indicating that also oxidation reactions take place. The reaction of Zn2Cp*2 (Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl) with K4Ge9 in en yielded the linear polymeric unit 1∞{Zn[μ2(η4:η1Ge9)]}2- with the first head-to-tail arrangement of ten-atomic closo-clusters. All anions are obtained and structurally characterized as [A(2.2.2–crypt)]+ salts (A = K, Rb). Copious computational analyses on a DFT-PBE0/def2-TZVPP/PCM level of theory confirm the experimental structures and support the stability of the two hypothetical ten vertex clusters fragments closo-[Ge9Zn]2– and (paramagnetic) [Ge9Zn]3–.
See Paper:
K. Mayer, L.-A. Jantke, S. Schulz, T. F. Fässler
Intermetalloid clusters: The retention of the Zn–Zn bond in [Ge9Zn–ZnGe9]6– and formation of [(Ge9Zn) –(Ge9)–(ZnGe9)]8– as well as polymeric 1∞[–(Ge9Zn)2––]