Decomposition of nitrous oxide by transition metal catalysts
Environmental issues catalysis such as the reduction of gaseous emissions from vehicles, stationary sources, and other industrial processes is one of the most important catalysis branches.
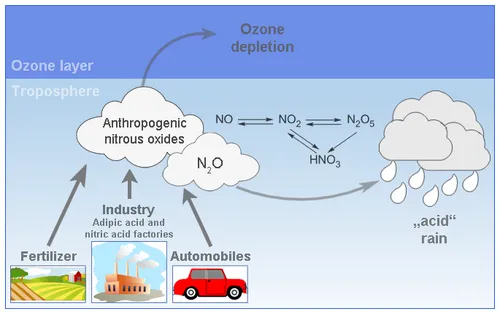
Carbon oxides (COx), nitrogen oxides (NOx), volatile organic compounds (VOC), and sulfur oxides (SOx) are common gaseous pollutants. Nitrogen oxides, NOx, are known to form nitric acid vapor and to cause ozone depletion in the stratosphere. They can react with common organic chemicals to form a wide variety of toxic products like nitroarenes and nitrosamines.
Nitrous oxide (N2O), a member of the nitrogen oxides, is an air pollutant and a greenhouse gas. It possesses a long lifetime of 110 – 150 years and a 310 times higher Global Warming Potential (GWP) than CO2. N2O is one of the main sources for acid rain formation in the atmosphere. It is also an undesirable by-product in exhaust gases of vehicles. Anthropogenic N2O sources are responsible for the increase of N2O in the atmosphere over the past centuries e.g. the agricultural sector, engine exhausts and the industrial production of nitric acid and adipic acid. It was reported that the global annual N2O concentration increased by approximately 19% from 1750 to 2007. Especially in the past 30 years, the increase of the N2O concentration has been observed to be faster than before.
Currently, methods for N2O abatement mainly involve thermal destruction, recycling of stream (for adipic acid or nitric acid production), and catalytic destruction (reduction or direct decomposition). Recycling of stream is mainly applicable on an industrial scale but catalytic decomposition of nitrous oxide is the most suitable method to destroy N2O emitted by vehicles or at low concentration levels. The mechanism of N2O decomposition depends on the type of catalyst and the reaction conditions.
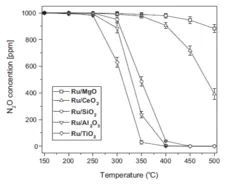
The main goal of our research is a deeper understanding of the influence of the support and reaction gases on catalytic N2O decomposition as well as improving transition metal catalyst performance.
Analytical tools:
-
Infrared spectroscopy (IR) of gaseous components
-
Temperature programmed techniques (TPR, TPD)
-
X-ray powder diffraction (XRD)
Contact:
Patrick Schlachta (patrick.schlachta(at)tum.de)