1. Transition metal-NHC complexes in oxidation catalysis
Iron is the most abundant transition metal. It is inexpensive, nontoxic and environmentally benign, which makes it an ideal candidate for catalysis. Iron-based catalysts can be applied in various fields, e.g. cross-coupling, hydrosilylation, (transfer-)hydrogenation and oxidation reactions.[1] Our group focuses on the development of transition metal-catalysts, particularly iron with polydentate NHC-ligands mimicking enzymes facilitating oxidation reactions. We have synthesized iron(II) complex 1 bearing a tetradentate bis(pyridyl-NHC)-ligand (NCCN), which belongs to the most active and selective homogenous catalysts in the formation of phenols from arenes (fig. 1).[2] Additionally, this catalyst is the first reported iron-NHC complex applied in epoxidation of olefins showing high activity and selectivity.[3]
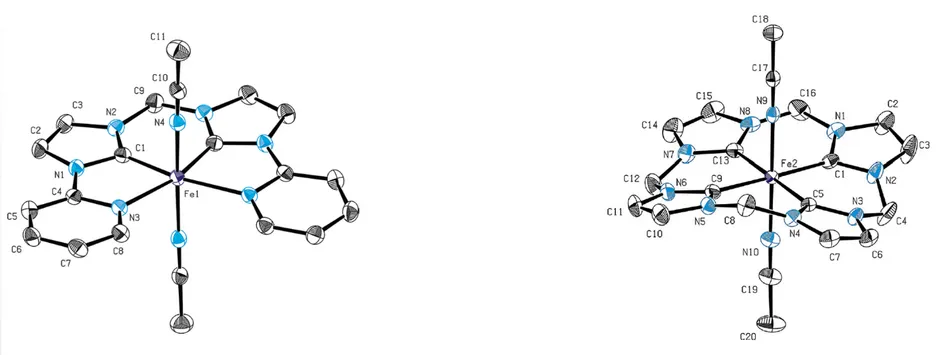
Further research led to the development of iron(II) and iron(III) 2 complexes bearing a methylene-bridged cyclic tetra-NHC-ligand (cCCCC) which show structural analogies with porphyrine-ligated iron complexes (fig. 1).[4] Iron(III) complex 2 shows remarkable activity in epoxidation catalysis using aqueous hydrogen peroxide as oxidant (TOF 183 000 h-1) considerably exceeding that of the optimized benchmark system methyltrioxorhenium (MTO) (TOF = 40 000 h-1).[5] While the corresponding iron(II) complex is also more active than MTO it is less active than compound 2, reaching a TOF of 50 400 h-1 and showing an induction period, probably due to in situ formation of the iron(III) species 2.[6] Our current focus lies on increasing the stability of these iron-based oxidation catalysts by ligand modification, e.g. by introducing functional groups at the methylene bridges opening the way to pentadentate “hangman”-type complexes or immobilization.[7] Other efforts towards iron complexes bearing non-imidazole derived NHC-ligands which exhibit even stronger donor capabilities are currently made in order to better stabilize intermediates with high oxidation states occurring during catalysis. Several other transition metals are under examination with related ligand systems.
Figure 1: ORTEP-style representation of cationic fragments of NCCN-iron(II) complex 1 (left) and cCCCC-iron(III) complex 2 (right). Thermal ellipsoids are shown at a 50% probability level. Hydrogen atoms, co-crystallized solvent molecules and anions are omitted for clarity.[6, 8]
2. Hydrogenation catalysis and hydrogen storage
Petroleum has been the most applied carbon feedstock in industry for many years. Due to the fast consumption of this limited feedstock and current public concerns about rapidly increasing CO2-levels in the atmosphere, associated with global warming, the need for a sustainable “green” carbon source arises. Wood-derived lignin appears to be promising for this purpose. In this field our research focuses on the development of homogenous water soluble catalysts facilitating the hydrogenation of aromatic compounds derived from lignin. Recently, we have reported the synthesis of water-soluble ruthenium(II) and osmium(II) complexes bearing a bidentate sulfonated bis-NHC-ligand. The ruthenium(II) compound is a precatalyst for the efficient complete hydrogenation of acetophenone and phenol, which were used as model substrates exhibiting oxygen-functionalization similar to biomass related arenes.[9]
Another result of the long term depletion of petroleum resources and the above mentioned environmental concerns is the demand for alternative and environmentally acceptable energy carriers. Dihydrogen (H2) is considered as a promising candidate, due to water being its only combustion product. H2, not available in significant amounts in its molecular form on Earth has to be produced on an industrial scale by steam reforming of natural gas, which has a high energy demand (and contributes to the release of additional CO2 as by-product). Furthermore, H2 storage, handling and transport have problematic aspects, which have to be overcome. A possible solution is the chemical storage of hydrogen using formic acid. Thus, we have developed water soluble rhodium complexes bearing sulfonated bis-NHC-ligands which not only effectively facilitate the reduction of bicarbonate to formate using H2 but also allow the hydrogen generation from formic acid at ambient conditions.[10]
Another field of research in hydrogenation catalysis we are focusing on is the synthesis of heterobimetallic complexes bearing anionic N-heterocyclic dicarbenes (NHDCs) and their application in bifunctional catalysis. Recently, in cooperation with Prof. Dr. Baratta (Università di Udine) we have reported Pd/Ru NHDC complex 3 where the Ru centers are coordinated by the abnormal moiety of the NHDC ligand and the normal moieties coordinate to the Pd center (fig. 2). This compound is the first reported catalyst that is active in tandem Suzuki Miyaura cross coupling/transfer hydrogenation reaction of bromoacetophenones and boronic acids with formation of biphenyl alcohols. The bimetallic species facilitates a higher selectivity than the combination of both monometallic species. Furthermore this tandem process reduces the number of synthetic steps towards biphenyl alcohols leading to less solvent consumption and an increase in overall yield.[11] Our current focus lies on the development of other heterobimetallic NHDC complexes facilitating different tandem reactions.
Figure 2: Structure of Pd/Ru NHDC catalyst 3 for tandem Suzuki-Miyaura/transfer hydrogenation reaction of bromoacetophenones and boronic acids.[11]
3. Other Aspects of our Research
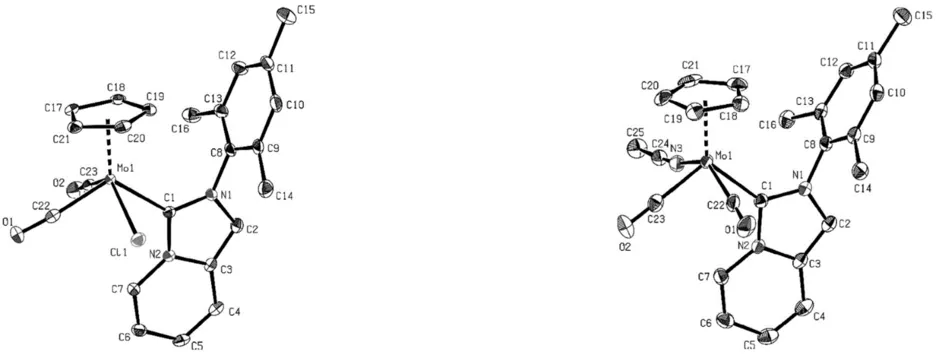
Our research in the broad field of homogenous catalysis not only focuses on metal NHC and (transfer-)hydrogenation catalysts but also includes the usage of molybdenum based compounds facilitating epoxidation reactions. We have developed new cyclopentadienyl molybdenum complexes 4 and 5 bearing a bicyclic imidazo[1,5-a]pyridine-3-ylidene ligands (fig. 3). These compounds (TOF up to 53 100 h–1) outperform most previously reported molybdenum-based epoxidation catalysts and even enable the epoxidation of challenging substrates.[12]
Figure 3: ORTEP-style representation of 4 and 5. Thermal ellipsoids are shown at a 50% probability level. Hydrogen atoms and anions (in case of 5) are omitted for clarity.[12]
Aside from molybdenum catalysts our group also works on catalysis using molecular rhenium compounds. Recently, we have reported that methyldioxorhenium (MDO) generated in situ by reduction of methyltrioxorhenium (MTO) facilitates the effective cleavage of C–O bonds of various lignin β-O-4 model compounds yielding in phenolic and aldehydic compounds. These results indicate, that MDO seems to be a promising candidate for the challenging depolimerization of lignin, which is considered to be a sustainable carbon feedstock of the future.[13] Several research projects are also performed in close co-operation with chemical companies in order to synthesize straight forward and efficient catalysts for larger scale application beyond purely academic considerations. Nevertheless, mechanistic and spectroscopic examinations are performed on these compounds and in these projects as well to fully understand and further improve these systems.
Literature
[1] K. Riener, S. Haslinger, A. Raba, M. P. Högerl, M. Cokoja, W. A. Herrmann, F. E. Kühn, Chem. Rev. 2014, 114, 5215-5272.
[2] A. Raba, M. Cokoja, W. A. Herrmann, F. E. Kühn, Chem. Commun. 2014, 50, 11454-11457.
[3] J. W. Kück, A. Raba, I. I. E. Markovits, M. Cokoja, F. E. Kühn, ChemCatChem 2014, 6, 1882-1886.
[4] M. R. Anneser, S. Haslinger, A. Pöthig, M. Cokoja, J.-M. Basset, F. E. Kühn, Inorg. Chem. 2015, 54, 3797-3804.
[5] P. Altmann, M. Cokoja, F. E. Kühn, Eur. J. Inorg. Chem. 2012, 2012, 3235-3239.
[6] J. W. Kück, M. R. Anneser, B. Hofmann, A. Pöthig, M. Cokoja, F. E. Kühn, ChemSusChem 2015, 8, 4056-4063.
[7] R. Zhong, A. C. Lindhorst, F. J. Groche, F. E. Kühn, Chem. Rev. 2017, 117, 1970-2058.
[8] a) A. Raba, M. Cokoja, S. Ewald, K. Riener, E. Herdtweck, A. Pöthig, W. A. Herrmann, F. E. Kühn, Organometallics 2012, 31, 2793-2800; b) S. Haslinger, J. W. Kück, E. M. Hahn, M. Cokoja, A. Pöthig, J. M. Basset, F. E. Kühn, Inorg. Chem. 2014, 53, 11573-11583.
[9] D. Jantke, M. Cokoja, M. Drees, W. A. Herrmann, F. E. Kühn, ChemCatChem 2013, 5, 3241-3248.
[10] D. Jantke, L. Pardatscher, M. Drees, M. Cokoja, W. A. Herrmann, F. E. Kühn, ChemSusChem 2016, 9, 2849-2854.
[11] M. J. Bitzer, F. E. Kühn, W. Baratta, J. Catal. 2016, 338, 222-226.
[12] A. Schmidt, N. Grover, T. K. Zimmermann, L. Graser, M. Cokoja, A. Pöthig, F. E. Kühn, J. Catal. 2014, 319, 119-126.
[13] R. G. Harms, I. I. E. Markovits, M. Drees, W. A. Herrmann, M. Cokoja, F. E. Kühn, ChemSusChem 2014, 7, 429-434.
Medicinal Chemistry
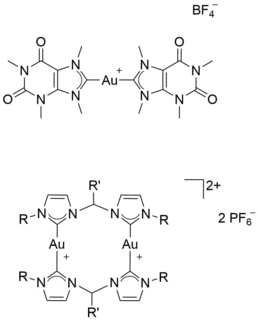
Since the discovery of cisplatin (cis-diamminedichloridoplatinum(II), [Pt(NH3)2(Cl)2]) in 1965 by B. Rosenberg and its approval as antitumor agent in 1978, it is mainly used to treat several types of cancer. However, cisplatin shows severe side effects such as neurotoxicity due to its cytotoxic effects not only against cancer cells but also against (mainly fast growing) healthy cells. Another problem is the development of resistances. A lot of research is dedicated to overcome the platinum-resistance, to decrease the side effects and increase the effects of the drug by improving the selective targeting of cancer cells to improve the patients’ quality of life and increase their life expectancy. Another prominent example for medicinal used inorganic compounds is Auranofin, a gold(I) thiolate phosphine complex that is used to treat rheumatoid arthritis. The high binding affinity of gold ions and sulfur leads to interactions of gold complexes and sulfur-containing peptides/enzymes like glutathione reductase (GR), thioredoxin reductase (TrxR) and cysteine proteases. If these enzymes are overexpressed on the surface of tumor cells, they represent a suitable target for cancer therapy using gold compounds.[1]
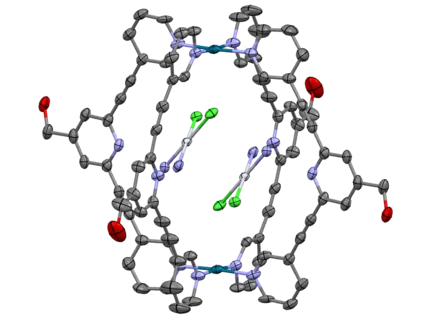
In collaboration with Prof. Dr. Angela Casini (Cardiff University) current research is focused on the synthesis of various mono- and bis-N-heterocyclic (NHC) carbene complexes of gold(I/III) as well as cyclometalated gold(III) compounds and their application. These compounds are characterized using NMR, MS and SC-XRD and evaluated in cell tests for their cytotoxic and antiproliferative effects.[2-4] In the field of supramolecular chemistry, also in co-operation with Prof. Casini, investigations on the modification of transition metal based M2L4 (M = metal, L = ligand) cage structures are done. Using these compounds as drug delivery vehicles for cisplatin, the side effects and the dose can be reduced, whereas the effects can be increased.[5-8]
Further research is done in collaboration with Prof. Dr. João Correia (University of Lisbon) in the area of peptide based transition metal complexes of gold and ruthenium. By combining cytotoxic metal complexes and known peptide ligands interacting with overexpressed receptors at the surface of cancer cells, also a more selectively targeted cancer therapy is investigated.[9]
Furthermore, parts of the biological evaluation of the potentially medicinal applicable compounds is realized in collaboration with Prof. Dr. S. Sieber (TUM) which resulted in publications in 2016 and 2017.[4, 8]
Literature
[1] F. E. Kühn and A. Schmidt, Chemie in unserer Zeit, 2017, 51, 86-95.
[2] Ö. Karaca, V. Scalcon, S. M. Meier-Menches, R. Bonsignore, J. M. J. L. Brouwer, F. Tonolo, A. Folda, M. P. Rigobello, F. E. Kühn and A. Casini, Inorganic Chemistry, 2017, 56, 14237-14250.
[3] S. Jürgens, F. E. Kühn and A. Casini, Curr Med Chem, 2018, 25, 437-461.
[4] J. Rieb, B. Dominelli, D. Mayer, C. Jandl, J. Drechsel, W. Heydenreuter, S. A. Sieber and F. E. Kühn, Dalton Transactions, 2017, 46, 2722-2735.
[5] A. Schmidt, A. Casini and F. E. Kühn, Coordination Chemistry Reviews, 2014, 275, 19-36.
[6] A. Schmidt, M. Hollering, M. Drees, A. Casini and F. E. Kühn, Dalton Transactions, 2016, 45, 8556-8565.
[7] A. Schmidt, V. Molano, M. Hollering, A. Pöthig, A. Casini and F. E. Kühn, Chemistry – A European Journal, 2016, 22, 2253-2256.
[8] F. Kaiser, A. Schmidt, W. Heydenreuter, P. J. Altmann, A. Casini, S. A. Sieber and F. E. Kühn, European Journal of Inorganic Chemistry, 2016, 2016, 5189-5196.
[9] E. M. Hahn, N. Estrada‐Ortiz, J. Han, V. F. C. Ferreira, T. G. Kapp, J. D. G. Correia, A. Casini and F. E. Kühn, European Journal of Inorganic Chemistry, 2017, 2017, 1667-1672.