Micropollutants and Pesticides
Chemical micropollutants (e.g., pesticides, pharmaceuticals & consumer care products) at low (sub-mg/L) concentrations are a most notorious environmental pollution of our time. In Germany and Austria, for example, pesticides are the second most important reason for the closure of drinking water wells (after nitrate) and pharmaceuticals in ground- and surface water are receiving increasing attention. Available methods to investigate micropollutant degradability in the environment, however, are limited: mass balances are difficult to close, metabolites are not always detectable and changes in concentrations alone do not provide conclusive evidence. Compound-specific isotope analysis can provide a complementary line of evidence. On the one hand, isotope ratios at natural abundance can be used as fingerprints to distinguish sources and commercial products. On the other hand, due to the isotope effects associated with environmental transformations, enrichment of heavy isotopes (e.g., 13C, 15N, 2H or 37Cl) in the original micropollutant can serve as the equivalent of an “isotopic footprint” which provides a direct proof of degradation, even if metabolites cannot be detected (Elsner et al., J. Environ. Monit. 2010). Using isotope ratios of multiple elements (dual isotope plots), it allows to identify different degradation pathways and provides a more promising fingerprinting of products from different origin.
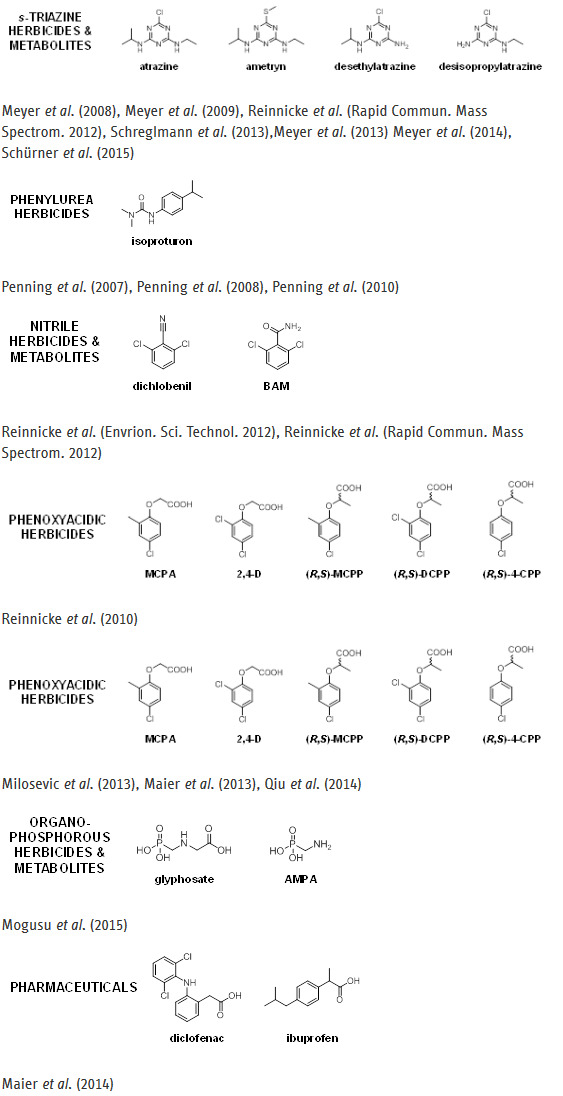
Micropollutants for which isotope analysis has been developed in our group:
Micropollutants for which isotope analysis is currently developed in a SINERGIA collaboration*:
* SINERGIA is a SNSF (Swiss National Science Foundation)-funded research project headed by the University of Neuchâtel in collaboration with the Helmholtz Zentrum München, the Swiss Federal Institute of Aquatic Science and Technology, and the Agroscope Reckenholz-Tänikon Research Station. The project focuses on the development of methods for isotope analysis of polar micropollutants and their use to detect degradation of pesticides directly in nature (lysometer studies at Agroscope, Zurich).
Publications:
- A. Melsbach, C. Torrentó, V. Ponsin, J. Bolotin, L. Lachat, V. Prasuhn, T. Hofstetter, D. Hunkeler, M. Elsner, Dual-Element Isotope Analysis of Desphenylchloridazon to Investigate its Environmental Fate in a Systematic Field Study - A Long-Term Lysimeter Experiment, Environ. Sci. Technol. 54 (2020), DOI: 10.1021/acs.est.9b04606
- C Lihl, B. Heckel, A. Grzybkowska, A. Dybala-Defratyka, V. Ponsin, C. Torrentó, D. Hunkeler, M. Elsner, Compound-Specific Chlorine Isotope Fractionation in Biodegradation of Atrazine, Environmental Science: Processes & Impacts 22 (2020), pp. 792 – 801; DOI: 10.1039/C9EM00503J
- Mogusu, E. O.; Wolbert, B. J.; Kujawinski, D. M.; Jochmann, M. A.; Elsner, M., Dual Element (15N/14N, 13C/12C) Isotope Analysis of Glyphosate and AMPA by Derivatization-Gas Chromatography Isotope Ratio Mass Spectrometry (GC/IRMS) Combined with LC/IRMS, Anal. Bioanal. Chem. 2015, 407, 5249-5260.
- C. Lihl, J. Renpenning, S. Kümmel, F. Gelman, H. Schuerner, M. Daubmeier, B. Heckel, A. Melsbach, A. Bernstein, O. Shouakar-Stash, M. Gehre and M. Elsner, Towards improved accuracy in chlorine isotope analysis: synthesis routes for in-house standards and characterization via complementary mass spectrometry methods, Anal. Chem. 91 (2019) pp. 12290-12297; doi: 10.1021/acs.analchem.9b02463
- C. Torrentó, R. Bakkour, G. Glauser, A. Melsbach, V. Ponsin, T. Hofstetter, M. Elsner, D. Hunkeler, Solid-phase extraction method for stable isotope analysis of pesticides from large volume environmental water samples, Analyst 144 (2019) pp. 2898-2908 doi: 10.1039/c9an00160c
- A. Melsbach, V. Ponsin, C. Torrentó, C. Lihl, T. Hofstetter, D. Hunkeler, M. Elsner, 13C and 15N isotope analysis of desphenylchloridazon by liquid chromatography isotope ratio mass spectrometry (LC-IRMS) and derivatization-gas chromatography isotope ratio mass spectrometry (GC-IRMS), Anal. Chem. 91 (2019) pp. 3412–3420; doi: 10.1021/acs.analchem.8b04906
- M. Gharasoo, M. Thullner, M. Elsner, Introduction of a new platform for parameter estimation of kinetically complex environmental systems, Environ. Modell. Softw. 98C (2017) pp. 12-20, DOI: 10.1016/j.envsoft.2017.09.005
- M. P. Maier, C. Prasse, S. G. Pati, S. Nitsche, Z. Li, M. Radke, A. Meyer, T. B. Hofstetter, T. A. Ternes, M. Elsner, Exploring Trends of C and N Isotope Fractionation to Trace Transformation Reactions of Diclofenac in Natural and Engineered Systems, Environmental Science & Technology, 50 (2016), pp 10933–10942, dx.doi.org/10.1021/acs.est.6b02104
- Schuerner, H.; Maier, M.; Eckert, D.; Brejcha, R.; Neumann, C.-C.; Stumpp, Ch.; Cirpka, O.; Elsner, M., Compound-Specific Stable Isotope Fractionation of Pesticides and Pharmaceuticals in a Mesoscale Aquifer Model; Environmental Science and Technology, 50 (2016) pp 5729–5739, http://dx.doi.org/10.1021/acs.est.5b03828
- Schürner, H. K. V.; Seffernick, J. L.; Grzybkowska, A.; Dybala-Defratyka, A.; Wackett, L. P.; Elsner, M., Characteristic Isotope Fractionation Patterns in s-Triazine Degradation Have Their Origin in Multiple Protonation Options in the s-Triazine Hydrolase TrzN, Environ. Sci. Technol. 2015, 49, 3490-3498.
- Meyer, A. H.; Dybala-Defratyka, A.; Alaimo, P. J.; Geronimo, I.; Sanchez, A. D.; Cramer, C. J.; Elsner, M., Cytochrome P450-catalyzed Dealklyation of Atrazine by Rhodococcus sp. strain NI86/21 Involves Hydrogen Atom Transfer rather than Single Electorn Transfer, Dalton Trans. 2014, 43, 12175-12186.
- Qiu, S.; Gözdereliler, E.; Weyrauch, P.; Lopez, E. C. M.; Kohler, H. P. E.; Sørensen, S. R.; Meckenstock, R. U.; Elsner, M., Small 13C/12C Fractionation Contrasts with Large Enantiomer Fractionation in Aerobic Biodegradation of Phenoxy Acids, Environ. Sci. Technol. 2014, 48, 5501-5511.
- Maier, M. P.; De Corte, S.; Nitsche, S.; Spaett, T.; Boon, N.; Elsner, M., C & N Isotope Analysis of Diclofenac to Distinguish Oxidative and Reductive Transformation and to Track Commercial Products, Environ. Sci. Technol. 2014, 48, 2312-2320.
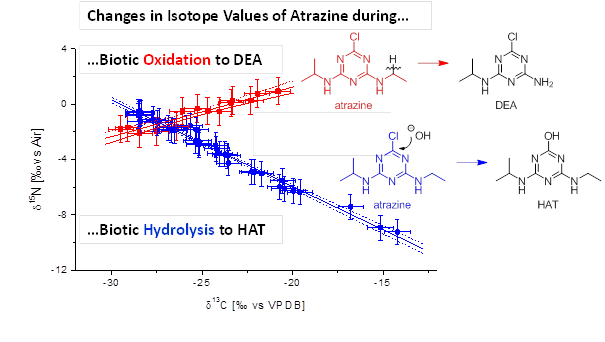
- Meyer, A. H.; Elsner, M., 13C/12C and 15N/14N Isotope Analysis to Characterize Natural Degradation of Atrazine: Evidence from Parent and Daughter Compound Values, Environ. Sci. Technol. 2013, 47, 6884–6891.
- Schreglmann, K.; Hoeche, M.; Steinbeiss, S.; Reinnicke, S.; Elsner, M., Carbon and Nitrogen Isotope Analysis of Atrazine and Desethylatrazine at Sub-µg/L Concentrations in Groundwater, Anal. Bioanal. Chem. 2013, 405, 2857-2867.
- Maier, M. P.; Qiu, S.; Elsner, M., Enantioselective Stable Isotope Analysis (ESIA) of Polar Herbicides, Anal. Bioanal. Chem. 2013, 405, 2825-2831.
- Milosevic, N.; Qiu, S.; Elsner, M.; Einsiedl, F.; Maier, M. P.; Bensch, H. K. V.; Albrechtsen, H.-J.; Bjerg, P. L., Combined Isotope and Enantiomer Analysis to Assess the Fate of Phenoxy Acids in a Heterogeneous Geologic Setting at an old Landfill, Water Research 2013, 47, 637-649.
- Reinnicke, S.; Juchelka, D.; Steinbeiss, S.; Meyer, A.; Hilkert, A.; Elsner, M., Gas Chromatography/Isotope Ratio Mass Spectrometry of Recalcitrant Target Compounds: Performance of Different Combustion Reactors and Strategies for Standardization, Rapid Commun. Mass Spectrom. 2012, 26, 1053-1060.
- Reinnicke, S.; Simonsen, A.; Sørensen, S. R.; Aamand, J.; Elsner, M., C and N Isotope Fractionation during Biodegradation of the Pesticide Metabolite 2,6-Dichlorobenzamide (BAM): Potential for Environmental Assessments, Environ. Sci. Technol. 2012, 46, 1447-1454.
- Penning, H.; Sørensen, S. R.; Meyer, A. H.; Aamand, J.; Elsner, M., C, N, and H Isotope Fractionation of the Herbicide Isoproturon Reflects Different Microbial Transformation Pathways, Environ. Sci. Technol. 2010, 44, 2372-2378.
- Reinnicke, S.; Bernstein, A.; Elsner, M., Small and Reproducible Isotope Effects during Methylation with Trimethylsulfonium Hydroxide (TMSH): A Convenient Derivatization Method for Isotope Analysis of Negatively Charged Molecules, Anal. Chem. 2010, 82, 2013-2019.
- Meyer, A. H.; Penning, H.; Elsner, M., C and N Isotope Fractionation Suggests Similar Mechanisms of Microbial Atrazine Transformation Despite Involvement of Different Enzymes (AtzA and TrzN), Environ. Sci. Technol. 2009, 43, 8079-8085.
- Penning, H.; Cramer, C. J.; Elsner, M., Rate-Dependent Carbon and Nitrogen Kinetic Isotope Fractionation in Hydrolysis of Isoproturon, Environ. Sci. Technol. 2008, 42, 7764-7771.
- Meyer, A. H.; Penning, H.; Lowag, H.; Elsner, M., Precise and Accurate Compound Specific Carbon and Nitrogen Isotope Analysis of Atrazine: Critical Role of Combustion Oven Conditions, Environ. Sci. Technol. 2008, 42, 7757-7763.
- Penning, H.; Elsner, M., Intramolecular Carbon and Nitrogen Isotope Analysis by Quantitative Dry Fragmentation of the Phenylurea Herbicide Isoproturon in a Combined Injector/Capillary Reactor Prior to GC Separation, Anal. Chem. 2007, 79, 8399-9405.