B. Heckel, M. Elsner.
Environmental Science & Technology (2021)
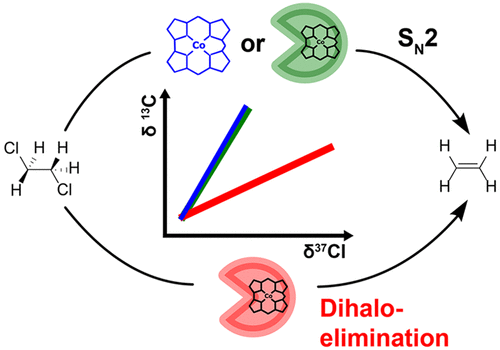
Abstract
Chlorinated alkanes are notorious groundwater contaminants. Their natural reductive dechlorination by microorganisms involves reductive dehalogenases (RDases) containing cobamide as a cofactor. However, underlying mechanisms of reductive dehalogenation have remained uncertain. Here, observed products, radical trap experiments, UV–vis, and mass spectra demonstrate that (i) reduction by cobalamin (vitamin B12) involved chloroalkyl–cobalamin complexes (ii) whose formation involved a second-order nucleophilic substitution (SN2). Dual element isotope analysis subsequently linked insights from our model system to microbial reductive dehalogenation. Identical observed isotope effects in reduction of trichloromethane by Dehalobacter CF and cobalamin (Dehalobacter CF, εC = −27.9 ± 1.7‰; εCl = −4.2 ± 0.‰; λ = 6.6 ± 0.1; cobalamin, εC = −26.0 ± 0.9‰; εCl = −4.0 ± 0.2‰; λ = 6.5 ± 0.2) indicated the same underlying mechanism, as did identical isotope effects in the reduction of 1,2-dichloroethane by Dehalococcoides and cobalamin (Dehalococcoides, εC = −33.0 ± 0.4‰; εCl = −5.1 ± 0.1‰; λ = 6.5 ± 0.2; cobalamin, εC = −32.8 ± 1.7‰; εCl = −5.1 ± 0.2‰; λ = 6.4 ± 0.2). In contrast, a different, non-SN2 reaction was evidenced by different isotope effects in reaction of 1,2-dichloroethane with Dehalogenimonas (εC = −23.0 ± 2.0‰; εCl = −12.0 ± 0.8‰; λ = 1.9 ± 0.02) illustrating a diversity of biochemical reaction mechanisms manifested even within the same class of enzymes (RDases). This study resolves open questions in our understanding of bacterial reductive dehalogenation and, thereby, provides important information on the biochemistry of bioremediation.