New Avenues in Main Group Chemistry
Simply put, chemistry is the study of valence electrons. As such, elements with the same number of valence electrons tend to behave in similar ways to each other but not completely analogously. The study of these differences is of great importance to fundamental chemists. Bonding is also of paramount interest, attempting to form different types of bonds between elements which at one point of history was thought not possible. A prime example are the unsaturated compounds of carbon; alkenes, alkynes etc. which are ubiquitous in nature. Now compared to its heavier analogue, silicon the same compounds (disilenes) don’t exist naturally. At the latter part of the last century a stable disilene was isolated and showed exceptionally different chemistry compared to its lighter cousin. One of the major research interests within the Inoue group is the synthesis and study of main group compounds exhibiting novel bonding/oxidation state/geometry to enrich the fundamental understanding of chemistry. Regarding main group chemistry the major interests of the Inoue group can be grouped into three key areas:
Low Valent Main Group Compounds
Main Group Compounds as Transition Metal Mimics
Our interests lie in trying to emulate bonding seen in the d-block, using p-block elements. We aim to invoke transition metal-like reactivity and bonding in main group elements. One notable example is our reported silylene carbonyl complex, a compound exhibiting Si-CO bonding reminiscent of transition metal complexes. Our goal is to further enrich this chemistry but examining such species and establishing differing reactivity compared to its d-block counterpart.
X. Liu, S. Dong, J. Zhu, S. Inoue, J. Am. Chem. Soc., 2024, 146, 2359. (10.1021/jacs.4c08171)
H. Zhu, A. Kostenko, J. A. Kelly, S. Inoue, Chem, 2024, 10, 1213. (10.1016/j.chempr.2024.01.007)
H. Zhu, A. Kostenko, D. Franz, F. Hanusch, S. Inoue, J. Am. Chem. Soc., 2023, 145, 1011. (10.1021/jacs.2c10467)
D. Reiter, R. Holzner, A. Porzelt, F. Frisch, S. Inoue, Nature Chem., 2020, 12, 1131. (10.1038/s41557-020-00555-4)
Synthesis of Heavy Main Group Analogues:
A key research area to manifest in recent times is that of heavy main group elements forming compounds analogous to their lighter congeners as well as transition metals. A key example is the synthesis of heavy multiple bonded compounds, which in earlier times were thought to be impossible to form. Isolation of such compounds has broadened our understanding of bonding and has elicited new and exciting reactivity. Instances of this from our group has included disilenes (Si=Si), silanones (Si=O), dialumenes (Al=Al), and alumenes (Al=C). The carbon analogues of these compounds are ubiquitous and one of research focus is the synthesis of heavy main group compounds that parallel those found in organic chemistry. Recent examples of this from our group include heavy nitriles ((NHC)P=SiR), heavy borata-alkenes ([R2Al=SiR2]-), heavy ketenyl anions ([RSi=C=O]-) and in terms of reactivity example of a “sila-Wittig” reaction (R2Si=O + Ph3P=CR2 → R2Si=CR2 + Ph3P=O).
Relevant Publications:
J. A. Kelly, A. Kostenko, S. Inoue, Nature Synth., 2025, 4, 1577-1586. (10.1038/s44160-025-00874-9)
F. J. Kiefer, A. Kostenko, R. Holzner, S. Inoue, J. Am. Chem. Soc., 2025, 147, 26663. (10.1021/jacs.5c07040)
M. E. Doleschal, A. E. Ferao, A. Kostenko, F. J. Kiefer, S. Inoue, Angew. Chem. Int. Ed., 2025, 64, e202422186. (10.1002/anie.202422186)
M. E. Doleschal, A. Kostenko, J. Y. Liu, S. Inoue, Nature Chem., 2024, 16, 2009. (10.1038/s41557-024-01618-6)
M. Ludwig, D. Franz, A. E. Ferao, M. Bolte, F. Hanusch, S. Inoue, Nature Chem., 2023, 15, 1452. (10.1038/s41557-023-01265-3)
S. Fujimori, A. Kostenko, R. Scopelliti, S. Inoue, Nature Synth., 2023, 2, 688. (10.1038/s44160-023-00283-w)
D. Reiter, P. Frisch, T. Szilvási, S. Inoue, J. Am. Chem. Soc. 2019, 141, 1699. (10.1021/jacs.9b09379)
P. Bag, A. Porzelt, P. J. Altmann, S. Inoue, J. Am. Chem. Soc. 2017, 139, 14384. (10.1021/jacs.7b08890)
Study of Low Oxidation State Main Group Compounds:
The isolation of low oxidation state compounds of p-block elements has triggered a renaissance in main group chemistry. Such compounds have help further of understanding of bonding and the electronic nature of elements. They have also expanded what we thought was possible regarding reactivity, such as the activation of inert bonds, believed only possible with transition metals. We are constantly discovering new aspects of main group chemistry with the isolation of novel p-block compounds. Our interests lie in low oxidation state group 14 (tetrylenes, tetryliumylidenes, ditetrenes) and group 13 (ditrianes, ditrienes) compounds.
Relevant Publications:
D. Sarkar, C. Weetman, D. Munz, S. Inoue, Angew. Chem. Int. Ed., 2021, 60, 3519. (10.1002/anie.202013423)
D. Reiter, R. Holzner, A. Porzelt, P. Altmann, P. Frisch, S. Inoue, J. Am. Chem. Soc., 2019, 141, 13536. (10.1021/jacs.9b05318)
T. Ochiai, T. Szilvási, D. Franz, E. Irran, S. Inoue, Angew. Chem. Int. Ed., 2016, 55, 11619. (10.1002/anie.201605636)
T. Ochiai, D. Franz, X. Wu, E. Irran, S. Inoue, Angew. Chem. Int. Ed., 2016, 55, 6983. (10.1002/anie.20162178)
D. Franz, E. Irran, S. Inoue, Angew. Chem. Int. Ed., 2014, 53, 14264. (10.1002/anie.201408709)
S. Inoue, C. Eisenhut, J. Am. Chem. Soc., 2013, 135, 18315. (10.1021/ja410528y)
S. Inoue, K. Leszszyńska, Angew. Chem. Int. Ed. 2012, 51, 8589. (10.1002/anie.201203257)
Catalytic Application of Main Group Compounds:
We wish to expand the fundamental knowledge gained from our research to find viable applications for low oxidation state main group compounds. The area most primed for this is that of catalysis, utilising earth abundant, non-toxic main group catalyst in important transformations. The inherent reactivity of low oxidation state main group compounds towards inert bonds opens up the possibilities for many functionalisation reactions. We are also interested in highly Lewis acidic main group compounds in catalysis. Our main driving force is synthesising highly abundant element catalysts that can compete (and eventually exceed) the effectiveness of exotic precious metal compounds, with a focus on silicon and aluminium-based complexes due to being the most abundant elements in the earth’s crust (after oxygen).
Relevant Publications:
X. Liu. A. Kostenko, M. D. D. Roy, T. Weng, S. Inoue, Nature Commun., 2025, 16, 8230. (10.1038/s41467-025-63460-9)
D. Niu, A. Kostenko, J. A. Kelly, D. Sarkar, H. Xu, S. Inoue, Chem. Sci., 2025, 16, 4014. (10.1039/D4SC07116F)
F. S. Tschernuth, T. Thorwart, L. Greb, F. Hanusch, S. Inoue, Angew. Chem. Int. Ed., 2021, 60, 25799. (10.1002/anie.202110980)
D. Sakar, C. Weetman, S. Dutta, E. Schubert, C. Jandl, D. Koley, S. Inoue, J. Am. Chem. Soc., 2020, 142, 15403. (10.1021/jacs.0c06287)
C. Weetman, A. Porzelt, P. Bag, F. Hanusch, S. Inoue, Chem. Sci., 2020, 11, 4817. (10.1039/D0SC01561J)
V. Nesterov, R. Baierl, F. Hanusch, A. E. Ferao, S. Inoue, J. Am. Chem. Soc., 2019, 141, 14576. (10.1021/jacs.9b08741)
C. Weetman, P. Bag, T. Szilvási, C. Jandl, S. Inoue, Angew. Chem. Int. Ed., 2019, 58, 10961. (10.1002/anie.201905045)
Main Group Clusters
There is fundamental interest in polyhedral cluster compounds as they can be seen as the intermediate between the fully oxidised element and its metallic form. The study of the intrinsic bonding mode found in discreet clusters will allow us better insight into delocalised and metal bonding. Our goal is the synthesis of homo- and heteroatomic cluster compounds and to explore their electronic nature and reactivity.
Relevant Publications:
H. Xu, M. M. D. Roy, A. Kostenko, J. A. Kelly, S. Fujimori, S. Inoue, Angew. Chem. Int. Ed., 2024, 63, e202404532. (10.1002/anie.202404532)
X. Zhao, T. Szilvási, F. Hanusch, J. A. Kelly, S. Fujimori, S. Inoue, Angew. Chem. Int. Ed., 2022, 61, e202208930. (10.1002/anie.202298930)
G. Dübek, F. Hanusch, D. Munz, S. Inoue, Angew. Chem. Int. Ed., 2020, 59, 5823. (10.1002/anie.201916116)
D. Franz, T. Szilvási, E. Irran, S. Inoue, Nature Comm., 2015, 6, 10037. (10.1038/ncomms10037)
Main Group Elements in Polymer Applications
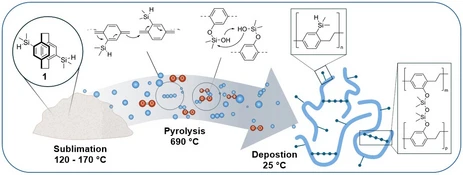
Main-group element functionalized polymers are gaining increasing importance due to their ability to introduce unique chemical, electronic, and structural properties into conventional polymer backbones. By expanding beyond traditional carbon-based functionalization, researchers can access entirely new classes of materials with tuneable characteristics. In particular, functionalization with elements from groups 13–15 of the periodic table offers significant potential. Incorporating boron, aluminium, phosphorus, or nitrogen functionalities, for example, can enhance thermal stability, modify electronic behaviour, improve processability, and enable applications in fields ranging from optoelectronics to advanced coatings.
At our chair, the first steps toward such innovative materials have been realized through the functionalization of poly(p-xylylene) (PPX) with silicon, germanium, and tin which lead to unique polymer properties in all three cases. These efforts demonstrate the potential of group 14 elements to systematically and predictably tailor polymer properties.
Building on these achievements, our current research explores further opportunities for main-group element incorporation across a broader range of polymer systems.
L. Bichlmaier, T. F. Teshima, A. Kostenko, G. Al Boustani, R. Wilhelm, S. Stigler, S. Tanaka, H. Onoe, B. Wolfrum, S. Inoue, Adv. Mater. Interfaces, 2024, 12 (10.1002/admi.202400701)
Silicon in Medicinal Applications
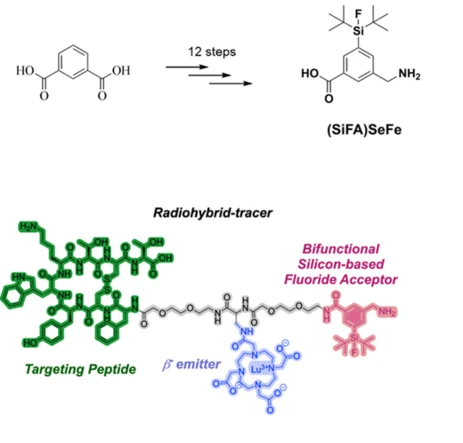
In an ever-aging society, it is becoming increasingly important to develop improved diagnostic methods and new pharmaceutical active ingredients. In molecular medicinal research, silicon is the element with an astonishingly huge gap between its high availability as a natural resource and its diminished use as an active ingredient. This is all the more disconcerting when one considers silicon's low toxicity and its direct relationship to carbon, the basic building block of life on Earth. Silicon-containing APIs (active pharmaceutical ingredients) have so far played a negligible role in medicine. However, isolated research has shown that the direct replacement of carbon atoms with silicon atoms in the pharmacophoric centers of APIs (the so-called "silicon switch") or the additional functionalization of established APIs with silicon-containing groups can have remarkable effects on the pharmacodynamics and pharmacokinetics of APIs. Regarding a related medical application, we recently reported the use of silicon fluoride complexes in radiomedicine for diagnostic and therapeutic purposes (i.e., SiFAs for 18F PET imaging in radiohybride-tracers for theranostic applications). Although known for 20 years, only a few successful SiFA tracers have been developed in the past. One of the main reasons for this is the negative impact on the hydrophilicity of the radiotracer after functionalization with the SiFA group. Therefore, our group developed the more hydrophilic and versatile (SiFA)SeFe, which can be supplemented with a chelator to complex a radioactive metal (e.g., 68Ga, 177Lu). Finally, conjugation with a biomolecule takes place according to the intended biological target structure. In this research topic, we combine the expertise of the Inoue group in organosilicon chemistry and the overall expertise in bioinorganic chemistry, as well as in radiopharmaceutical chemistry of the department to explore the broad field of molecular silicon compounds in medical applications. We focus on the exploration of organosilicon-functionalized APIs for cancer therapy and for antibacterial treatments. Moreover, we promote our studies on SiFAs and radiohybdrid-tracers for theranostic applications together with the Casini group. The results will significantly advance the previously underdeveloped research field of medicinal silicon chemistry.
S. Deiser, S. Fenzel, V. König, M. Drexler, L. M. Smith, M. E. George, R. Beck, T. H. Witney, S. Inoue, A. Casini, J. Med. Chem. 2024, 67, 14077
Reviews
Over the years our group has composed many reviews, targeting key areas of interest for our research:
N-Heterocyclic Carbene-Phosphinidenes in Main Group Chemistry - H. Zhu, S. Inoue, Chem, 2025, 11, 102649. (10.1016/j.chempr.2025.102649)
Tetryliumylidene Ions in Synthesis and Catalysis - S. Stigler, S. Fujimori, A. Kostenko, S. Inoue, Chem. Sci., 2024, 15, 4275. (10.1039/D3SC6452B)
Uncharged Lewis Superacidic Silicon Complexes - Perfluoropinacolato-stabilized Systems for Homogenous Catalysis - F. Hanusch, D. Franz, S. Inoue, Chem. Lett., 2024, 53, upae232. (10.1093/chemle/upae232)
Dearomatization of C6 Aromatic Hydrocarbons by Main Group Complexes - H. Zhu, S. Fujimori, A. Kostenko, S. Inoue, Chem. Eur. J., 2023, 29, e202301973. (10.1002/chem.202301973)
Carbon Monoxide in Main-Group Chemistry - S. Fujimori, S. Inoue, J. Am. Chem. Soc., 2022, 144, 2034. (10.1021/jacs.1c13152)
Recent Advances of Group 14 Dimetallenes and Dimetallynes in Bond Activation and Catalysis - F. Hanusch, L. Groll, S. Inoue, Chem. Sci., 2021, 12, 2001. (10.1039/D0SC03192E)
Small Molecule Activation by Two‐Coordinate Acyclic Silylenes - S. Fujimori, S. Inoue, Eur. J. Inorg. Chem., 2020, 3131. (10.1002/chem.202000479)
Cationic Complexes of Boron and Aluminum ‐ An Early 21st Century Viewpoint - D. Franz, S. Inoue, Chem. Eur. J., 2019, 25, 2898. (10.1002/chem.201803370)
Experimental Realisation of Elusive Multiple-bonded Aluminium Compounds: A New Horizon in the Aluminium Chemistry - P. Bag, C. Weetman, S. Inoue, Angew. Chem. Int. Ed., 2018, 57, 14394. (10.1002/anie.201803900)
The Road Travelled: After Main-group Elements as Transition Metals - C. Weetman, S. Inoue, ChemCatChem., 2018, 10, 4213. (10.1002/cctc.201800963)
NHCs in Main Group Chemistry - V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt, S. Inoue, Chem. Rev., 2018, 118, 9678. (10.1021/acs.chemrev.8b00079)
Advances in Phosphasilenes - V. Nesterov, N. C. Breit, S. Inoue, Chem. Eur. J., 2017, 23, 12014. (10.1002/chem.201700829)
Applications of N-heterocyclic Imines in Main Group Chemistry - T. Ochiai, D. Franz, S. Inoue, Chem. Soc. Rev., 2016, 45, 6327. (10.1039/C6CS00163G)