Our Research
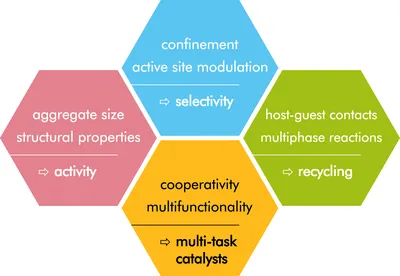
Our research is rooted in applied catalysis using molecular catalysts, as well as colloidal metal catalysts for the conversion of small molecules and industrially relevant products. We are focused on epoxidations, CO2 activation and selective hydrogenation of alkynes. Our research strategy is driven by two main motifs: (1) to understand and to control the catalyst activity and product selectivity by tuning the catalyst environment, and (2) to find simple ways to separate the product and to reuse the catalyst by working in biphasic media and by using supramolecular assemblies of the catalyst.
Surface-active Ionic Liquids as Micellar Catalysts
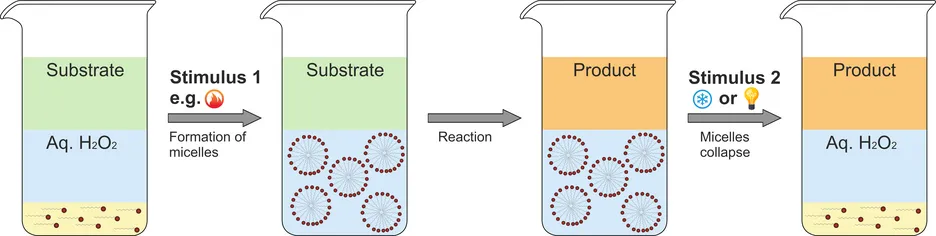
Imidazolium-based surface-active ionic liquids (SAIL) can aggregate in water to form micellar structures, which can be used to solibilize organic molecules in water. Epoxidations of olefins in the aqueous phase are the target of choice, since catalyst separation and reusability is a notorious problem for molecular epoxidation catalysts. We use SAIL catalysts with catalytically active element-oxo anions, which are only active when the counterion is a surfactant, but not in case of simple metal cations. The aggregation and the charged interface allow for catalyst-H2O2 contacts. For example, the perrhenate [ReO4]- and even the nitrate anion can efficiently activate H2O2 via H-bond interactions allowing for olefin epoxidation in water. Our current activities are focused on the development of cheap, metal-free SAIL catalysts for epoxidations, and on the control of aggregation by cations which respond to external stimuli, such as temperature or light for an application in continuous reactors beyond the lab scale (in cooperation with Andreas Jess, University of Bayreuth).
DFG-funded research grant for the project "Hydrophobic Ionic Liquids as Micellar Catalysts for the Epoxidation of Olefins".
Activation of hydrogen peroxide by the nitrate anion in micellar media, F. Schmidt, B. Zehner, M. Kaposi, M. Drees, J. Mink, W. Korth, A. Jess, M. Cokoja,* Green Chem. 2021, 23, 1965.
Ionic liquid surfactants as multitasking micellar catalysts for epoxidations in water, F. Schmidt, B. Zehner, W. Korth, A. Jess, M. Cokoja,* Catal. Sci. Technol. 2020, 10, 4448.
Ionic Liquids as Micellar Agents in Perrhenate-catalysed Olefin Epoxidation, J. Schäffer, M. Alber, W. Korth, M. Cokoja, A. Jess,* ChemistrySelect 2017, 2, 11891.
Supramolecular Ion Pairs
Supramolecular Ion Pairs - or SIPs - are formed from peptide-inspired amidoammonium receptors (in cooperation with Jason Love, University of Edinburgh) and element-oxo anions for epoxidation catalysis. We exploit the cation-anion interactions to wrap the hydrophilic anions in an organic shell, rendering them soluble in non-polar organic solvents. Thereby, we carry out biphasic epoxidations: the SIP catalyst operates in the substrate phase, while transferring the oxidant, hydrogen peroxide, from the aqueous phase. We are investigating the SIP formation from cheapest possible organic molecules: an amide, an ammonium cation and an anions, such as sulfate. We study the SIP structure (in cooperation with Alexander Pöthig, TUM) the control and reversibility of deconstructing the SIP structure in order to bring the SIP components into the aqueous phase for the sake of facile product separation.

Catalytic epoxidation by perrhenate through the formation of organic-phase supramolecular ion pairs, M. Cokoja,* I. I. E. Markovits, M. H. Anthofer, S. Poplata, A. Pöthig, D. S. Morris, P. A. Tasker, W. A. Herrmann, F. E. Kühn,* J. B. Love,* Chem. Commun. 2015, 51, 3399.
Activation of Carbon Dioxide
We study the the catalytic activity of molecular organocatalysts for the cycloaddition of carbon dioxide with epoxides. We use metal-free tandem catalysts consisting of a protic/electrophilic catalyst - an imidazolium cation (see for example the structure of an imidazolium bromide catalyst below) or an alcohol for epoxide activation, and a nucleophilic halide. We adjust the acidity of the proton, aiming at strengthening the bond to the epoxide and a more facile ring opening. The goal is to convert CO2 to an added-value product and to avoid a carbon footprint by decreasing the reaction temperature with using metal-free, cheap and recyclable catalysts.
Hydroxy-Functionalized Imidazolium Bromides as Catalysts for the Cycloaddition of CO2 and Epoxides to Cyclic Carbonates, M. H. Anthofer, M. E. Wilhelm, M. Cokoja,* M. Drees, W. A. Herrmann, F. E. Kühn, ChemCatChem 2015, 7, 94.
Cycloaddition of CO2 and epoxides catalyzed by imidazolium bromides at mild conditions: influence of the cation on catalyst activity, M. H. Anthofer, M. E. Wilhelm, M. Cokoja,* I. I. E. Markovits, A. Pöthig, J. Mink, W. A. Herrmann, F. E. Kühn, Catal. Sci. Technol. 2014, 4, 1749.
Cycloaddition of CO2 and Epoxides using Pentaerythritol and Halides as Dual Catalyst System, M. E. Wilhelm, M. H. Anthofer, M. Cokoja,* I. I. E. Markovits, W. A. Herrmann, F. E. Kühn, ChemSusChem 2014, 7, 1357.
Surfactant-stabilized Metal Colloids for Hydrogenations
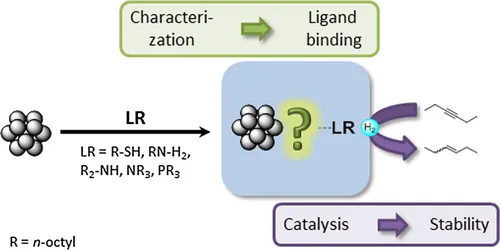
We investigate the influencing factors for controlling the selectivity of the hydrogenation of aklynes to olefins in homogeneous phase. We use colloidal metal catalysts (Pd, Pt) stabilized by ionic and σ-donor surfactants and vary their steic and electronic properties to learn about the influence on activity and selectivity. In cooperation with the Fischer team@AMC we also study the effect of alloying with a secon, non-catalytic metal such as aluminum and gallium, on the activity and selectivity of the catalyst.
Steric and Electronic Effects of Phosphane Additives on the Catalytic Performance of Colloidal Palladium Nanoparticles in the Semi‐Hydrogenation of Alkynes, L. Staiger, T. Kratky, S. Günther, O. Tomanek, R. Zbořil, R. W. Fischer, R. A. Fischer, M. Cokoja,* ChemCatChem 2021, 13, 227.
High stability of thiol-protected colloidal platinum nanoparticles with reduced ligand coverages in the hydrogenation of 3-hexyne, P. Wand, E. Kratzer, U. Heiz, M. Cokoja,* M. Tschurl,* Catal. Commun. 2017, 100, 85.
Functionalization of small platinum nanoparticles with amines and phosphines: Ligand binding modes and particle stability, P. Wand, J. D. Bartl, U. Heiz, M. Tschurl,* M. Cokoja,* J. Colloid Interface Sci. 2016, 478, 72.