Abstract of the full publication available here:
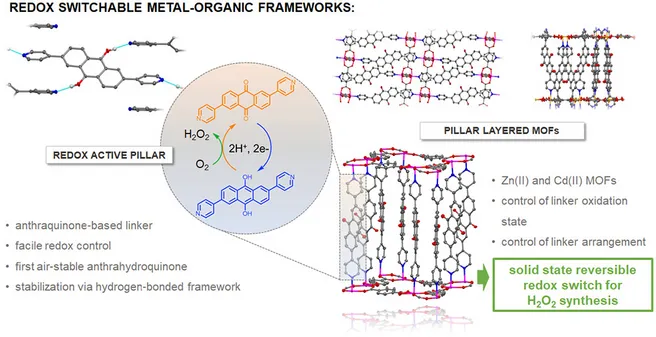
A dipyridyl-substituted anthraquinone (2,6-di(pyridin-4-yl)-9,10-anthraquinone, DPAq) was incorporated as a redox-active linker molecule into crystalline coordination networks. The oxidation state of the organic linker can be selectively controlled prior to framework formation and furthermore be maintained in the solid state. Hydrogen bonding is identified to be a substantial stabilization factor. Additionally, it is shown that the anthraquinone–anthrahydroquinone redox pair can be switched reversibly even after incorporation in the solid state by a thermal treatment/soaking procedure—going along with the formation of hydrogen peroxide from molecular oxygen (air) during the oxidation process.