Research Areas
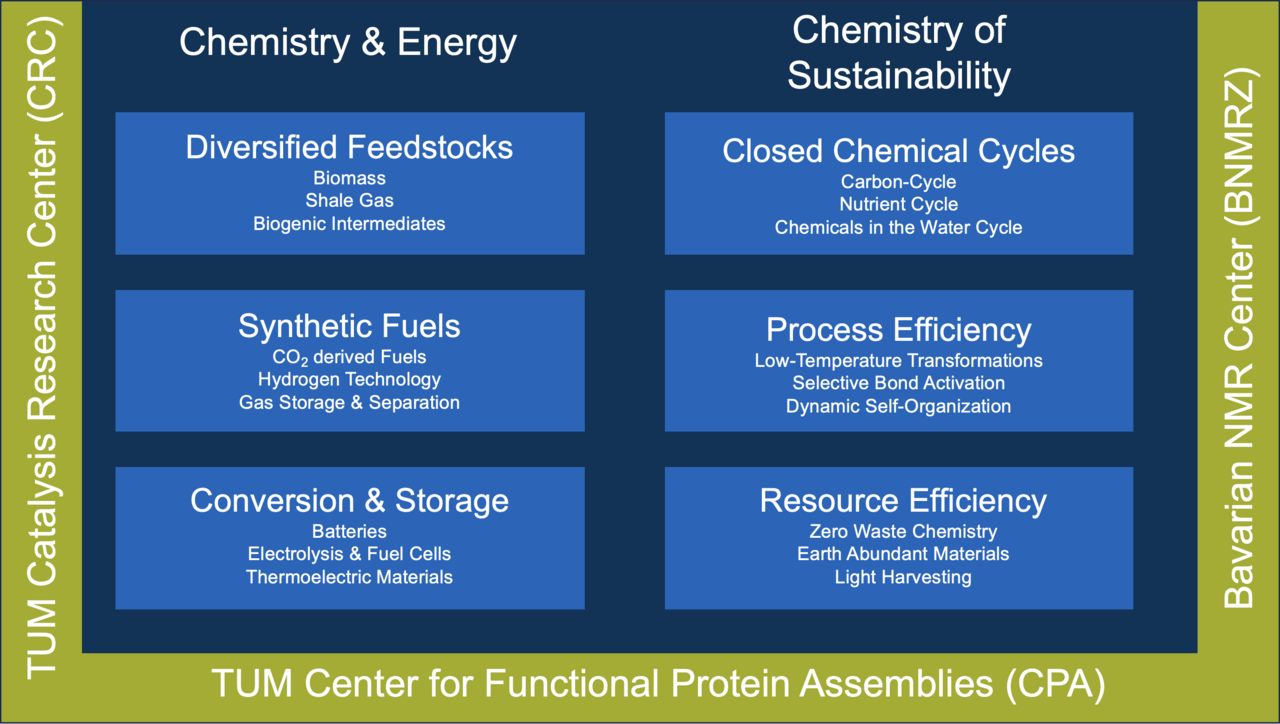
Our research areas can be divided according to two key aspects: “Chemistry and Energy” and “Chemistry of Sustainability”. Through our research, we try to address the most challenging issues of society’s present and near future. The associated research institutes on our campus also play an active role in our endeavour.
Chemistry and Energy
To satisfy society’s enormous energy demand, new concepts must be developed to store and harness energy in chemical bonds. Our Department of Chemistry addresses a wide range of feedstocks, synthetic fuels and storage systems. Research spans the investigation of microscopic chemical processes, the production of novel materials, and technology development.

En route to a fully renewable material cycle, diversified feedstocks beyond oil must be tapped. This requires the development of efficient chemical conversion processes for feedstocks like shale gas as a bridge technology. Beyond these fossil resources, biomass acts as an alternative for the production of energy carriers. The development of renewable biogenic intermediates links research in the field of energy and sustainability.
Research groups involved: Brück, Hinrichsen, Strunk

The conversion of abundant water and carbon dioxide offers the potential for large scale production of synthetic fuels. Targets include hydrogen and hydrocarbons like methanol. Novel solutions for gas separation and storage are integral to the associated technologies.
Research groups involved: Fischer, Günther, Hinrichsen, Inoue, Köhler, Strunk

Sustainably generated electricity necessitates the development of efficient chemical systems for the storage and release of energy on demand. Routes comprise electrolyzers and fuel cells, batteries and thermoelectric materials.
Research groups involved: Bucher, Elsner, Fässler, Kühn, Gasteiger, Hinrichsen, Nilges, Ortmann, Rupp, C. Stein, H. Stein, Strunk, Willinger
Chemistry of Sustainability
Our planet Earth is energetically an open system but strictly closed in the availability of matter. Within these constraints, the chemistry of the future must respond to the needs of society. Thus, we need to meet the requirements imposed by limited resources while avoiding the emission of pollution to keep a balanced environment.

Many current industrial processes are open chemical cycles as exemplified by CO2 emissions or inadequate resource disposal. Although chemistry can provide key solutions to close chemical cycles, this potential is far from being realized today. Research in the fields of carbon, nutrient and chemicals in water cycles is pursued to challenge this issue.
Research groups involved: Brück, Elsner, Gädt, Hinrichsen, Nilges, Strunk

Contemporary chemical processes suffer from a lack of selectivity, generate undesired by-products and consume an excessive amount of energy. Catalysis is a key technology that addresses these challenges of process efficiency and also opens unprecedented routes to novel molecular structures. Key targets regarding process efficiency are: Low Temperature Transformations, Selective Bond Activation and Dynamic Self-Organization.
Research groups involved: Casini, Bach, Boekhoven, Elsner, Fischer, Günther, Heiz, Hinrichsen, Hintermann, Inoue, Kühn, Köhler, C. Stein, H. Stein, Strunk, Willinger

Despite limited availability, current society relies on excessive use of resources. This implies that existing processes need to be revised in terms of resource efficiency. Excess waste streams and limited availability of crucial resources force us to rethink the economy of chemical reactions to get to a zero waste chemistry. This includes the examination of earth abundant materials and the use of light harvesting systems.
Research groups involved: Bach, Boekhoven, Bucher, Elsner, Fässler, Glaser, Gädt, Günther, Hauer, Heiz, Hintermann, Inoue, Kühn, Nilges, Ortmann, Rupp, C. Stein, H. Stein, Strunk, Willinger