Ambient Pressure Photocatalysis (e-conversion)
Aim and Motivation
Photocatalytic water splitting and alcohol reforming offer promising pathways for hydrogen production to curb the currently dominating steam reforming of fossil fuels in the long term. However, a lack of mechanistic understanding impedes the implementation of photocatalytic processes at industrial scale since the structural complexity of commonly used powder catalysts imposes difficulties on establishing structure-reactivity relationships. In contrast, fundamental studies on well-defined planar model catalysts in ultra-high vacuum (UHV) have already provided a mechanistic understanding of heterogeneously catalyzed photoreactions on a molecular scale. However, a direct transfer to applied conditions requires experimental verification, as unconsidered effects (known as the pressure and material gap) may lead to outcomes different to those of model systems. Therefore, we developed two experimental setups providing different environments to investigate the surface chemistry of a photocatalytic system at ambient pressure.
Photocatalysis at the Gas-Solid Interface: Our µ-Photoreactor Setup
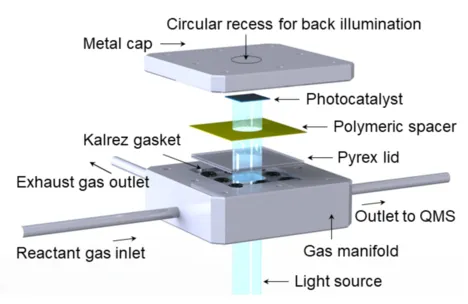
This µ-photoreactor was developed for the evaluation of planar model catalysts like single crystals or epitaxially grown semiconductor thin films at ambient conditions. The reaction volume of only 10.5 µL is accomplished by placing a polymer sealing between the catalyst and a Pyrex lid whose UV-Vis transparency allows illumination with an interchangeable light source. The channels integrated into the lid by lithography maintain the gas feed at 1000 mbar at the reactor access. At the outlet, a capillary transmits the entire gas flow directly to a quadrupole mass spectrometer enabling sensitive and time-resolved reaction monitoring. The precise dimensions of the capillary not only ensure a pressure drop of nine orders of magnitude but also define the low molecular flow through the reactor. This reactor design enables catalyst characterization also after the reaction.
Our µ-photoreactor is integrated into a setup infrastructure where the reactant gas feed is generated either by directly flowing gaseous components via mass-flow-controllers or by saturating a carrier gas with a liquid compound at a defined partial pressure. Using this setup, we could already show that the photo-oxidation of different alcohols over bare as well as co-catalyst loaded TiO2 (P25) powders under the exclusion of oxygen results in similar product selectivities as in UHV. While this µ-photoreactor setup is designed for the investigation of planar catalysts in a well-defined environment, it also enables the measurement of low amounts of powdered photocatalysts (µg scale).
Photocatalysis at the Liquid-Solid Interface: Our Liquid Phase Setup
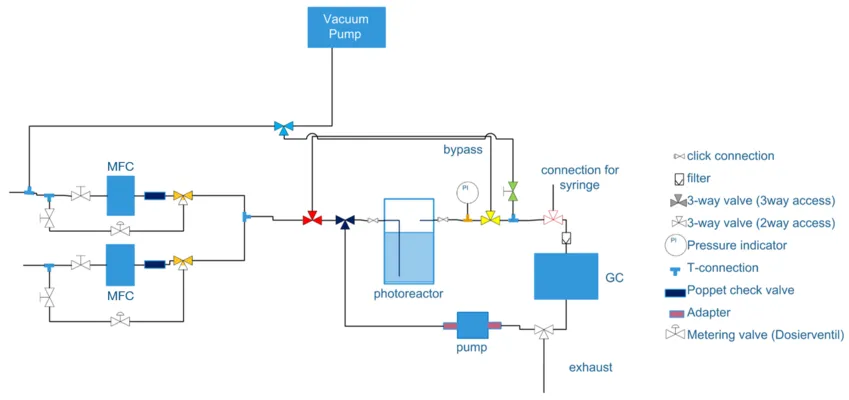
The developed liquid phase setup is specifically tailored for the comprehensive characterization of liquid and gas phase photoproducts in small sample concentrations by an online gas chromatograph (GC). The setup consists of an exchangeable photoreactor that is connected to a gas line system, where different inert or reactant gases can be introduced into the reactor by mass flow controllers (MFCs) or metering valves. The headspace of the photoreactor can be analyzed continuously under an inert gas flow. This mode is suitable for highly active powder catalysts. Alternatively, the headspace gas phase is circulated and thereby enriched with gaseous reaction products. This is of great benefit for the investigation of model planar catalysts such as single crystals or thin films. The photoreactor can be illuminated with different light sources, such as UV-LEDs at 365 nm or solar simulators. The GC is equipped with a universal thermal conductivity detector (TCD), a reducing gas detector (RGD, particularly suited for the analysis of low amounts of H2) and a flame ionization detector coupled with a methanizer (FIDM) for detection of organic compounds including CO and CO2. In addition to the headspace of the reactor cell, the liquid phase is analyzed after the catalytic measurements by injecting a small amount directly onto the column via a syringe. The setup could also be further equipped with a mass spectrometer (MS) for isotope-labelling studies. As the reactor itself is fully replaceable and enables the use of standard Schlenk tubes or other reaction cells, this setup is also suitable for collaborative experiments on photocatalytic and photoelectrocatalytic systems.
So far, we have tested our setup in the oxygen evolution reaction (OER) on BiVO4 photoanodes and investigated the hydrogen evolution reaction (HER) from alcohols on titanium dioxide powders loaded with different metals as co-catalysts. Besides these collaborative projects, our main goal is to discover reaction mechanisms and key properties of photocatalysts in order to make their performance relevant for application.
Laboratory and Scientists
- Lucia Mengel
- Paula Neumann
- Martin Tschurl (Coordination)
- Ueli Heiz
Collaborations (non-TUM)
Funding
- DFG Cluster of Excellence e-conversion