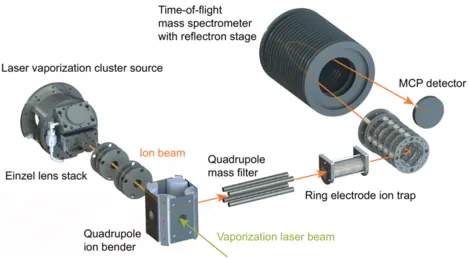
Studying Reaction Mechanisms of Isolated Metal Clusters with Small Molecules
Our Strategy
To control the outcome of reactions, e.g., in selective catalysis, it is imperative to understand the underlying reaction mechanisms. In applied systems, however, the complexity of the materials and the chemical environment makes it difficult or even impossible to elucidate reactions on a molecular level. In particular in heterogeneous catalysis, methods investigating the catalysts during the reaction (“operando”) quickly face significant limitations and inherently have unwanted side-effects (e.g., damage by electrons or hard radiation), whose contribution to the reaction has to be ruled out. Instead, we apply a different strategy by studying well-defined systems under well-defined conditions. Here, gas-phase studies of ion-molecule reactions are ideally suited to gain fundamental insights into reaction pathways and intermediates. This way, the active center in metal (oxide) clusters is fully known and possible reaction schemes and relevant properties of the catalyst can be identified, which are usually unknown in heterogeneous catalysis. Studying the reactions under multi-collision conditions may therefore provide valuable information about catalytic processes on the nanoscale.
Our Approach
Bare clusters of a metal of interest are produced in a laser vaporization cluster source, where they can also be chemically modified by pulses of a reactant gas (e.g. by O2 to obtain oxides). The charged clusters are then guided to a quadrupole mass filter, where the species of choice is separated from the unwanted rest. Only these clusters are then stored in a ring-electrode ion trap for a defined reaction time with the desired reactants (e.g., CH4), which can be introduced in the desired concentration. After that, the reaction is quenched by the extraction of all charged reaction products into a reflectron time-of-flight mass spectrometer, allowing the identification of reaction products and intermediates and the determination of their abundance. By varying the reaction time in the ring-electrode ion trap, reaction compositions for different reaction times are obtained, allowing the determination of underlying reaction schemes and reaction kinetics. Finally, the variation of the reactant concentration, reaction temperature, changing the clusters’ charge state and their chemical modification (e.g., the oxidation) enables a comprehensive understanding of the chemistry of the metal cluster and the reaction, in particular when the data is compared with theoretical calculations (usually DFT).
Our Current Project
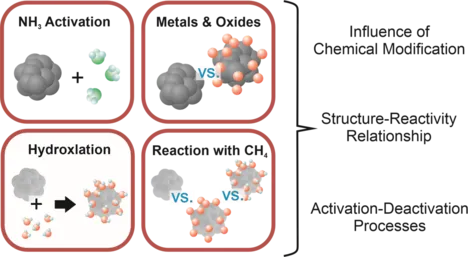
We are part of the CRC 1441 TrackAct, in which we strongly collaborate with different groups from the Karlsruhe Institute of Technology (KIT) and the Deutsches Elektronen-Synchrotron (DESY). The project’s goal is to track the active sites in heterogeneous catalysis, utilizing the knowledge transfer from model systems to real catalytic systems by increasing the complexity of the systems step-by-step. Our part is to provide information about the driving forces, potential reaction pathways, and important relevant properties of potential catalysts, which include oxidation states of clusters, their sizes, charge states on a surface tuned by a support material, and important structural motifs of the catalyst, and reaction intermediates. These insights are then transferred to more realistic systems to improve the catalytic properties of the systems. This way, we are elucidating the activation of ammonia using platinum and platinum oxide clusters. Since ammonia is seen as a potential, carbon-free alternative fuel source preventing its emission and over-oxidation to NOx will be important for running processes environmentally friendly in the future. Another reaction investigated is the activation of the greenhouse gas CH4 by Pd-catalysts, which is difficult because of the molecule's peculiar properties. The goal for both reactions is to enable the targeted design of efficient and powerful catalysts, for which an understanding of the intrinisic reactvity of clusters ist key.
Laboratory and Scientists
- Flora Siegele
- Martin Tschurl (Coordination)
- Ueli Heiz
Collaborations
- Different groups from KIT and DESY (https://www.trackact.kit.edu/)
Prof. K.H. Bowen (Johns Hopkins University, Baltimore)
Project Funding
Deutsche Forschungsgemeinschaft (DFG) via CRC 1441
Further Reading
- Activation of CH4, NH3, and N2 by Tantalum Ions, Clusters and Their Oxides: What Can Be Learnt from Studies of Ions in the Gas Phase
- Direct Coupling of Methane and Carbon Dioxide on Tantalum Cluster Cations
- Room-Temperature Methane Activation Mediated by Free Tantalum Cluster Cations: Size-by-Size Reactivity
- Catalytic Non-Oxidative Coupling of Methane on Ta8O2+