Abstract of the full text in Catalysis Science & Technology available here:
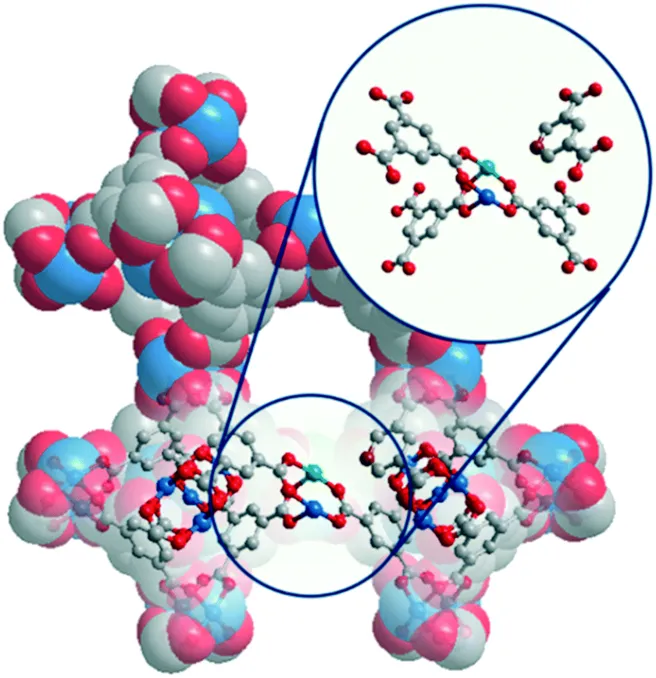
A series of Cu(I)-enriched metal–organic frameworks (MOF) of the type CuBTC (BTC = benzene-1,3,5-tricarboxylate) was prepared by a mixed-linker defect engineering technique, namely substituting a portion of a parent linker with truncated pyridine-3,5-dicarboxylate (PyDC) in the synthesis process. The reduced carboxyl coordination sites and the emerged Lewis basic pyridyl sites of PyDC spawned mixed-valence Cu(I)–Cu(II) paddlewheels (PWs) in the defect-engineered CuBTC (DE-CuBTC) structure. Cu(I)-enriched DE-CuBTC shows significantly enhanced catalytic performance for the click reaction of azide–alkyne cycloaddition by accelerating the rate determining step of Cu(I)–acetylide intermediate formation. To further evaluate the catalytic activity of Cu(I)-enriched DE-CuBTC for reactions involving a Cu(I)–acetylide intermediate, the A3 coupling reaction of phenylacetylene, paraformaldehyde and piperidine was studied as well. This study shows that defect engineering is an effective editing tool of catalytic active sites in catalysts.