Asymmetric catalytic fluorination
Work on the topic of asymmetric catalytic fluorination has been performed during my PhD thesis with Prof. Antonio Togni, ETH Zurich.
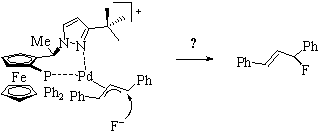
A feasibility study in1997/8 on the fluorination of certain 1,3-diphenyl allyl substrates revealed that a catalytic allylic fluorination was not possible in the system investigated (Chimia 2001, 55, 801; Eur. J. Inorg. Chem. 2006, 1397). Later, however, Katcher und Doyle were able to realize a catalytic asymmetric allylic fluorination reaction in a more suitable model system (JACS 2010, 132, 17402).
We have realized an asymmetric catalytic electrophilic fluorination, using the reagent F-TEDA (Banks) in combination with the Seebach/Narasaka TADDOL-titanium Lewis-acids (Angew. Chem. Int. Ed. 2000, 39, 4359). The model substrates used in that study (alpha-alkylated beta-keto esters) have also been asymmetrically chlorinated (Helv. Chim. Acta 2000, 83, 2425). They have since been used by many groups as model substrates for investigating asymmetric catalytic alpha-functionalization reactions (halogenation, hydroxylation, amination).
