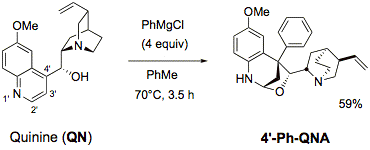D - Asymmetric organocatalysis
In asymmetric organocatalysis, reactions are catalyzed by (usually) small chiral molecules. We have studied asymmetric additions of alcohols to electron-poor alkenes (oxa-Michael reactions) and explored the use of Cinchona alkaloids and their derivatives as chiral organocatalysts. We have also studied reaction mechanisms of such reactions.
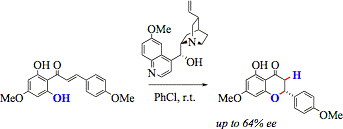
D-1 - Asymmetric catalytic oxa-Michael cyclizations
Asymmetric catalytic additions of alcohols to Michael-acceptors (oxa-Michael reactions) are little developed because of the limited nucleophilicity of the alcohols.
We have investigated the asymmetrix cyclization of 2-hydroxychalcones to flavanones as model of the biosynthetic chalcon isomerase reaction (Eur. J. Org. Chem. 2007, 5886; Eur. J. Org. Chem. 2012, 5573).
Additional work has been performed in order to elucidate the mechanism of a specific cinchona alkaloid catalyzed oxa-Michael cyclization (Chem. Eur. J. 2013, 19, 2311).
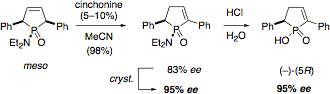
D-2 - Asymmetric organocatalytic olefin isomerization
We have found an asymmetric organocatalytic isomerization reaction for the double bond in meso-3-phospholenes, using cinchona alkaloids as catalysts, to give chiral 2-phospholenes in up to 83% ee (Adv. Synth. Cat. 2008, 350, 1469).
Further conversion of the 2-phospholenes to the C2-symmetric phospholane 1 (Fiaud's acid), a useful starting material for chiral phospholane ligand synthesis, has been realized (Synthesis 2013, 45, 308).

D-3 - Derivatisation of Cinchona alkaloids
Lithium alkyls add to the 2'-position of the quinoline of non-protected Cinchona alkaloids (quinine, quinidine, cinchonine, cinchonidine), opening easy access to 2'-aryl- and -alkyl-substituted Cinchona alkaloids.
Reactions of the alkaloids with Grignard reagents surprisingly lead to a new class of cinchona aminals with notable structures (Angew. Chem. Int. Ed. 2007, 46, 5164).