CoVRapid - Rapid detection of antibodies to SARS-CoV-2 in human blood by flow-based microarray immunoassays
OBJECTIVE
The world is kept in suspense by the COVID-19 pandemic. While on the one hand, first countries started vaccination campaigns in late 2020, new threats arise from the emergence of mutations in the genome of SARS-CoV-2. Therefore, a thorough serosurveillance will be helpful in the future to monitor immunity in the population. But some challenges for serosurveillance methods are also apparent: many typically employed antibody tests have several drawbacks like the need for specialized staff and equipment, long time expenditure or low sensitivity. Additionally, methods to selectively detect neutralizing antibodies will be needed and antibodies have to be tested for their protective force against different mutated variants of SARS-CoV-2. Therefore, we are developing different immunoassays on the microarray platform Microarray Chip Reader (MCR) that target these challenges:
- Immunoassay to detect the presence of IgG antibodies to different antigens from SARS-CoV-2
in serum and plasma
- Combined immunoassay for the sequential detection of IgG and IgM antibodies to SARS-CoV-2
- Microarray-based neutralization assays for the detection of neutralizing SARS-CoV-2 antibodies
- Avidity assay for SARS-CoV-2 antibodies
- Quantitative detection of antibodies to SARS-CoV-2 and endemic coronaviruses
- Extended immunoassay for the detection of SARS-CoV-2, influenza and other respiratory disease antibodies
METHODS
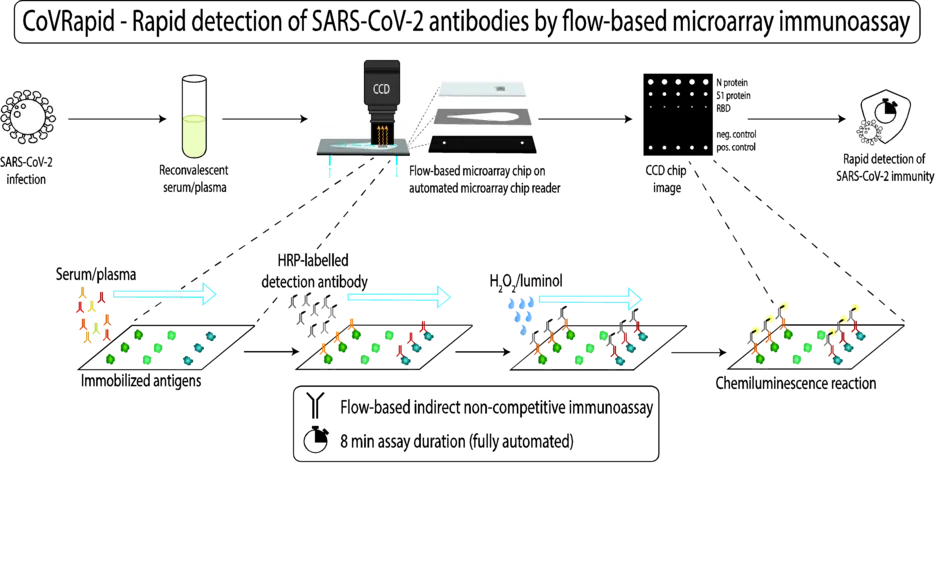
The used assay principle for most of the planned assays is a flow-based indirect non-competitive immunoassay on the MCR 3 or the newest microarray chip reader generation, MCR-R. Antigens are covalently immobilized on microarray chips, binding of specific antigens from blood samples and subsequent detection with horseradish-peroxidase labelled detection antibodies and chemiluminescence substrates hydrogen peroxide and luminol is done in a flow-based manner. The whole assay is carried out fully automated and within a very short timeframe.
INVOLVED PHD STUDENTS
M. Sc. Julia Klüpfel
PARTNERS
Heinz-Nixdorf-Chair for Biomedical Electronics at TranslaTUM (Prof. Oliver Hayden)
Institute of Virology (Prof. Ulrike Protzer)
Institute of Molecular Immunology (Prof. Percy Knolle)
GWK Präzisionstechnik GmbH
ISAR Bioscience GmbH
FINANCIAL SUPPORT
Bayerische Forschungsstiftung (AZ-1438-20C)