New Publication in Sensors
J.Neumair, M. Elsner and M. Seidel.
Sensors (2022)
Abstract
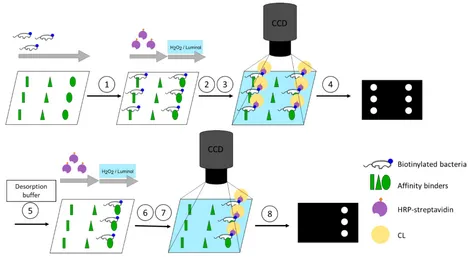
Affinity describes the non-covalent but selective interaction between an affinity binder (e.g., proteins, antibiotics, or antibodies) and its counterpart (e.g., bacteria). These affinity binders can serve to detect bacteria and respond to the need for selective concentration via affinity chromatography for trace analysis. By changing the pH value or salt and protein contents, affinity bindings can be reversed, and bacteria can be recovered for characterisation. Analytical microarrays use multiple affinity binders immobilised on the surface in a distinct pattern, which immensely reduces screening time for the discovery of superior binding motifs. Here, flow-based microarray systems can inform not only about binding, but also about desorption. In this work, we pioneer a screening assay for affinity binders against both gram-positive and negative bacteria based on an automated flow-based chemiluminescence (CL) microarray. Biotinylation of model organisms E. coli and E. faecalis enabled labelling with horseradish-peroxidase-coupled streptavidin, and detection with CL. Polymyxin B, an antibiotic against gram-negative bacteria, was found to bind both E. coli and E. faecalis. Simultaneous screening for desorption methods unexpectedly revealed methyl alpha-D-mannopyranoside as a promising buffer for desorption from Polymyxin B. This proof-of-principle study shows that our new platform greatly facilitates the screening of new affinity binders against bacteria, with promise for future automation.